Introduction: The Bruton tyrosine kinase inhibitor (BTKi) zanubrutinib has recently been approved for treatment of chronic lymphocytic leukemia (CLL). Zanubrutinib induces durable remissions in most patients with CLL, and treatment is typically given as continuous monotherapy, until disease progression or toxicities prohibit further use. We developed time-limited therapy with zanubrutinib in combination with rituximab as an alternative to long-term BTKi monotherapy. Here, we report early results from the first 17 treatment-naïve patients with CLL who enrolled in this ongoing phase 2 trial (NCT04458610) between July 2020 and April 2023 at MD Anderson Cancer Center.
Patients and Study Design: Patients with treatment-naïve CLL requiring therapy per iwCLL criteria receive zanubrutinib 160 mg orally twice daily and rituximab (375 mg/m 2 intravenously) on days 1, 8, 15, 22 of cycle 1, and then on day 1 of cycles 2 - 6 (one cycle is 28 days). Patients who achieve a complete remission (CR) by iwCLL 2018 criteria after 6 cycles of combination therapy continue with zanubrutinib alone for another 6 cycles. Patients who achieve a partial remission (PR) or stable disease (SD) after 6 cycles continue for another 6 cycles of the combination. Patients who achieve a CR after 12 cycles will receive 6 additional cycles of zanubrutinib alone, and then stop treatment. Patients who achieve a PR or SD after 12 cycles will continue zanubrutinib alone for 12 cycles and the stop therapy after a total of 24 cycles. After this time-limited therapy, patients transition to observation and can resume therapy if they relapse and fulfill iwCLL criteria for salvage therapy. The primary objective of the trial is to determine the proportion of patients who have treatment-free remission 6 months after discontinuation of zanubrutinib. Secondary objectives are to determine the length of treatment-free remission, identify clinical factors associated with long versus short remissions, and to evaluate the efficacy of re-treatment with zanubrutinib plus rituximab in patients who relapse.
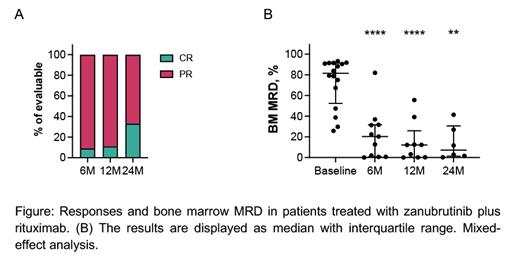
Results: The median age of the patients was 68 years (range, 54 - 77 years), 47% had unmutated IgHV and 41% advance stage disease (RAI stage III and IV). The median baseline absolute lymphocyte count (ALC) and β2 microglobulin were 51.9x10 9/L (range, 1.7 - 231.0x10 9/L) and 3.9 mg/L (range, 2.5 - 6.8 mg/L), respectively. After a median follow-up of 13 months (range, 0.5 - 29 months), all patients remain on study, and the estimated one-year progression-free (PFS) and overall survival (OS) is 100%. Seven patients completed therapy (1 patient after 12 cycles, 6 patients after 24 cycles) and have moved on to observation (median observation time post-therapy 4.5 months). So far, we have not observed any progression events after treatment discontinuation. Overall response rate is 100%, with 9% CR and 91% PR after 6 months (n=11), 11% CR and 89% PR after 12 months (n=9), and 33% CR and 67% PR after 24 months of therapy (n=6). The median levels of bone marrow involvement by CLL cells, quantified by flow cytometry, declined from 81.6% at baseline (range, 25.8 - 93.2%, n=16) to 20.3% after 6 cycles (range, 0.07 - 82%, n=11, p<0.0001), to 12.1% after 12 cycles (range, 0.04 - 55.60%, n=9, p<0.0001), and to 7.23% after 24 cycles (range, 0.2 - 41.4%, n=6, p=0.001). Grade ≥3 adverse events (AE) were observed in 9 patients (52.9%), which included hypertension (n=4) and decreased neutrophil counts (n=3). The most frequent grade 1-2 AE were decreased neutrophil (59%) and platelet counts (47%), bruising (41%), chills, increased creatinine, arterial hypertension, nausea (35% each), fatigue, and headache (29% each). No atrial fibrillation events were observed.
Conclusion: This early analysis indicates that time-limited therapy with zanubrutinib and rituximab is well tolerated and induces remissions with a major reduction in bone marrow disease burden in patients with treatment-naïve CLL. The first 7 patients who stopped therapy so far have not progressed, but longer follow-up in more patients is needed to determine the durability of responses after therapy discontinuation, and how those compare to more intensive CLL combination regimens.
Disclosures
Burger:Janssen: Other: Speaker fees and Travel Support; Abbvie, Beigene: Research Funding; AstraZeneca, Pharmacyclics: Other: Advisory Board, Research Funding. Ferrajoli:Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; GenMab: Research Funding; Genetech: Honoraria; Janssen: Honoraria. Wierda:Loxo Oncology, Inc./Lilly: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; Pharmacyclics LLC: Research Funding; Miragen: Research Funding; Genentech: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; AbbVie: Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Numab THerapeutics: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; Accutar Biotechnology: Research Funding; GlaxoSmithKline: Research Funding; Janssens Biotech Inc: Research Funding; Janssens Biotech: Research Funding; Juno Therapeutics: Research Funding; Nurix THerapeutics: Research Funding; Cyclacel: Consultancy, Research Funding; GSK/Novartis: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Sunesis: Research Funding; KITE Pharma: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal